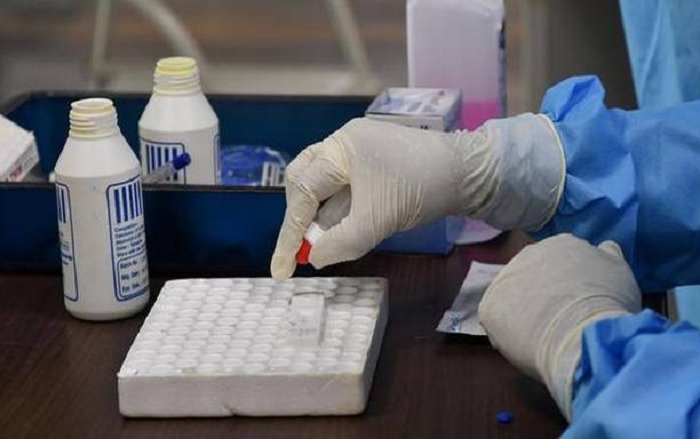
This is yet another news story underplayed by Big Media, and being reported by us. A Delhi HC judgment showed that ICMR agreed to pay 2.5 times the price for Corona rapid test kits, imported from China, to help private traders to loot the public exchequer.
Public interest must outweigh private gain, the Court observed. And Justice Najmi Waziri slashed price for every kit by 33 per cent from Rs 600 to Rs 400 per test, still allowing 60 percent profit. Normally this should have hit the headlines, but it was not the case.
Instead of probing why it took place, and taking action against those responsible, the Govt. launched an anti-China tirade, sought to cover up the misdeeds, very much like Trump did in USA.
The media helped the Govt. In this sordid episode rather than exposing the powers that be, the Big media,played Fox News, and facilitated the cover up and anti-China frenzy.
“ Acting on faulty anti-body testing kits, the Indian Council of Medical Research (ICMR), on Apr 25 Monday canceled the order of 15 lakh rapid anti-body test kits from China. The two Chinese firms – Guangzhou Wondfo Biotech and Zhuhai Livzon Diagnostics – which were given the order – have supplied kits which have produced inaccurate results, the body cited. Earlier in the day, ICMR had issued an advisory to states across the country halting the use of rapid antibody tests procured from two Chinese manufacturers.”
This was a widely reported, sensational report blaming China of supply of faulty anti-body testing kits. And an anti-China tirade was launched by the media, most of them obfuscating the issues involved, and avoiding a serious analysis of the same.
Only a few media outlets, some online, are a little objective, as can be seen below, but they are lost in the midst of an anti-China tirade.
The issues, at least some of them, need to be put in proper perspective. And dates are of some importance in this story.
businesstoday.in reported a Delhi High Court order of April 24 with this headline :
Massive 145% profiteering exposed in coronavirus rapid anti-body test kits (RAT kits) sold to ICMR.
Dispute between importer and distributor unravels super profits; Delhi HC on April 24strikes down sale price by 33 per cent. (Updated Report on April 27, 2020).
thenewsminute.com (TNM) April 27 report headlined it candidly :
ICMR agreed to pay 2.5 times the price for rapid test kits, Delhi HC judgment shows
Following the judgment, the ICMR now says it has not paid a single rupee, and the remaining order has been canceled.
Thus the HC order was on April 24, and ICMR canceled the order for 15 lakh kits on April 25, after it was exposed in the HC order. Their being faulty was a dubious reason.
businesstoday.in report gave highlights of the Court order thus :
RAT Kits were being imported from China’s firm Wondfo. Its landed price, including air freight (Rs 20), to importer Matrix Labs is Rs 245 per test ICMR placed an order to buy 5 lakh kits on March 27, 28 @Rs 600 (plus GST) per testImporter Matrix Labs sells to distributor Rare Metabolics Life Sciences at Rs 400 per test Rare Metabolics contracted to ICMR at the government approved rate of @Rs 600 (plus GST) 2.76 lakh have been delivered to ICMR; remaining 2.24 lakh tests are about to land Rare Metabolics intends to sell 1 million kits; order was placed with Matrix for 5 lakh kits Matrix Labs has an order to supply 50,000 kits to Tamil Nadu through dealer Shan Biotech and Diagnostics at Rs 600 per test.
Thus landed price was Rs 245. Importer Matrix Labs (ML) sells to distributor Rare Metabolics Life Sciences (RMLS) at Rs 400 per test, thus profiting by Rs 155 per kit. RMLS sells to ICMR at Rs 600 plus GST, a rate approved by ICMR, thus profiting at the rate of Rs. 200 per test.
The profit, but for the HC order, would have been Rs. 2325 lakhs for the importer, and Rs. 3000 lakh for the distributor, as per the rate approved by ICMR, the Govt agency. ICMR placed an order for 15 lakh kits, later canceled.
Because of a dispute between them, and because of the order by Justice Najmi Waziri, the whole deal fell through.
TNM reported:
In fact, because the ICMR set the price at Rs 600 per kit, even state governments like the government of Tamil Nadu are paying this exorbitant amount per kit…
The AP govt too was forced into such a position by the ICMR; so it had placed a condition in its order: If any state pays a lower rate, only that will be paid.
“In view of the above, the kits/test should be sold at a price not beyond Rs. 400/- per kit/test inclusive of GST, ” Justice Najmi Waziri said in his Order, and explained why Rs 400 :
“The Court is of the view that a profit mark-up of Rs. 155/- i.e 61% on the landed cost price of Rs. 245/- is much on the higher side and in any case more than sufficient for the seller…”
“The country is going through an unprecedented medical crisis affecting public order. People have been cloistered in their homes or constrained to stay wherever they were on 24th March 2020. The economy is virtually at a standstill for the last one month…. more kits/tests should be made available urgently at the lowest cost, for carrying out extensive tests throughout the country,” the HC said.
At such a juncture…
“Public interest must outweigh private gain. The lis between the parties should give way to the larger public good. In view of the above, the kits/test should be sold at a price not beyond Rs. 400/- per kit/test inclusive of GST,” Justice Najmi Waziri ordered.
Thus Justice Waziri slashed price for every kit by 33 per cent from Rs 600 to Rs 400 per test.
Delhi High Court Judge Muralidhar was transferred in a hurry in February last when he gave an order – because the CJ was on leave, the case was posted to him – related to Delhi communal riots. Now there has been a lockdown and cases were heard by video-conferencing mode. We do not know what befalls Justice Najmi Waziri; it was a Single Judge Bench.
Of the said order for five lakh, the Court was informed that 2.76 lakh had already been delivered to ICMR and the remaining 2.24 lakh tests were expected to be delivered very shortly after the consignment lands in India.
Further, the Court noted that for the kits, the ICMR had to shell out an amount of Rs 30 crores plus GST, “despite there being no value addition to the imported medical material.”
Though originally planned order was for 15 lakh kits, now canceled, this Court dispute pertained to a part of that, 5 lakh kits/tests:
It was case wherein agreements were arrived at, and the ICMR had approved the rates; the dispute was only about when payments should be made, not about price and profits.But the Judge went into that and gave the order. The litigants and ICMR apparently did not anticipate that.
Public interest must outweigh private gain, the Court observed : The dispute between the parties should give way to the larger public good.
“In view of the above, the kits/test should be sold at a price not beyond Rs. 400/- per kit/test inclusive of GST.”
TNM reported :
Govt defends, says no money was paid…
Meanwhile, the government has released a press statement on the pricing of rapid testing kits and stated that out of four tenders, the cheapest one was chosen. (Thus even a bigger loot was contemplated. Those who know how tendering is manipulated can see through the game.)
The government statement admitted that out of companies who offered to supply the kits, 2 companies (Biomedemics and Wondfo) were identified for procurement as both had the requisite international certifications.
The government, even after the Court exposure, justified the traders saying that since it was a “first ever effort by any Indian agency to procure such kits,” the “rate quoted by the bidders was the only reference point.”
“It needs to be stressed that ICMR has not made any payment whatsoever in respect of these supplies. Because of the due process followed (not going for procurement with 100% advance amount), GoI does not stand to lose a single rupee,” the press release by Ministry of Health and Family Welfare said.
Explaining why they didn’t import directly from Wondfo, the release said, “ICMR also tried to procure the kits directly from Wondfo company in China through CGI. However, quotation received from direct procurement had issues related to logistics, time-lines, dollar-basis etc..”
The real reason : It was all for the share, or the loot by the intermediaries, or middle men. And that is how Indian state serves comprador capitalism, now being turned into crony capitalism, and this goes on even at times of calamities like the Covid-19.
But for the Court order, all this loot would not have been revealed. And Modi regime would have continued as untainted in publicity.
That is the transparency level of the regime. And a largely pliant media. Meanwhile, the Indian Council of Medical Research on Monday issued a revised advisory for states, asking them to stop using rapid testing kits and return them all to the suppliers.
Two kinds of tests, for two different purposes
“The results have shown wide variation in their sensitivity, despite early promise of good performance for surveillance purposes. In view of this, states are advised to stop using these kits procured from the above mentioned companies and return them to be sent back to the suppliers,” the ICMR statement said.
ICMR added that the states should continue to rely on RT-PCR throat or nasal swabs for the diagnosis of COVID-19.
Here lies the catch. China clarified the issues which were sought to be obfuscated by ICMR.
There are two kinds of tests, for two different purposes.
RT-PCR(Real Time -Reverse Transcription Polymerase Chain Reaction ) test, is elaborate, Lab -based, is meant to diagnose and confirm the cases. But ten times costlier, Rs.4500 per test, fixed by Govt for Private hospitals.
Rapid anti-body test kits (RAT kits) are meant for surveillance purposes, for epidemiological study, not for diagnosis. It is fast, takes 20-30 minutes, and is far cheaper (Rs 245 landed cost, Rs 400 fixed by the Court).
15 lakh Chinese RAT kits were ordered by ICMR for surveillance purposes. As Govt. said it was “first ever effort by any Indian agency to procure such kits.”
Their being used on a mass scale all over India, without proper training of personnel in their maintenance and use is obviously riddled with problems.
www.newindianexpress.com 18th April 2020 reported:
ICMR asks states to use rapid antibody tests for surveillance in COVID-19 hotspots
ICMR Director-General Dr Balram Bharagava listed the protocol for using the ‘rapid antibody test’ in hotspot area for epidemiological studies and surveillance.
Stating it was critical to understand few key facts while using the rapid antibody tests, Bhargava said gold standard frontline test for COVID-19 diagnosis is real-time PCR based molecular test, which is aimed at early virus detection and the rapid antibody test cannot replace the frontline test.
“The rapid antibody test is a supplementary tool to assess the prevalence of the diseases within a specific area/perimeter. It will only be of utility after a minimum of seven days of onset of symptoms.”
“Data about these rapid tests is emerging and understanding of their utility for diagnosis is still evolving. The rapid test kits are useful for epidemiological studies and surveillance purposes,” he said. The test has to be done under strict medical supervision.
Dr. Arvind Kumar, noted lung surgeon of Delhi explained :
“ How well these tests work depends on several factors, including the time for the onset of illness, the concentration of virus in the specimen, the quality of the specimen collected from a person, and how it is processed, and the precise formulation of the reagents in the test kits…”
“ Additionally, false positive results – that is a test showing that a person is infected when they are not- could occur…” for instance if a person has common cold…
Likewise, a person who tested negative earlier, can came out positive later…Rapid tests wont address such problems….
(PTI report in Economic Times of April 14)
To cover up the profiteering deal the ICMR allowed, attempts were made to blame China.
China explained these things:
business-standard.com reported on April 29, 2020, with the heading Covid rapid antibody test kits row: China hits out at ‘individuals’ in ICMR :
The Chinese government has said criticism of the testing kits was “not based on facts” and has charged that it was “unfair and irresponsible for certain individuals to label Chinese products as ‘faulty’ and look at issues with preemptive prejudice”.
It said in a statement, issued late on April 27, that both companies had stressed that their Covid-19 rapid antibody test kits had obtained certification from the National Medical Products Administration of China (NMPA), meet the quality standards of China and exported countries, and were validated and approved by ICMR through the National Institute of Virology (NIV), Pune.
(NIV has been the sole competent and authentic body in India)
The kits produced by the firms had been exported to and well recognised in many countries in Europe, Asia, and Latin America, the statement added.
“Any operation that is not carried out by professionals in accordance with the product specifications will lead to the testing accuracy variations.
“ICMR also made it clear that rapid antibody test kits should only be used for surveillance purposes instead of replacing RT-PCR test to diagnose and confirm the cases, and States are advised to strictly abide by the methods and purpose of the usage according to ICMR’s clear instructions,” the statement said, warning that if the kits were used for purposes they were not intended to serve, the results they would show would be faulty.
The statement added: “we hope the Indian side could respect China’s goodwill and sincerity, strengthen communication with relevant Chinese companies based on facts, and resolve it reasonably and properly.”
These things were earlier confirmed by ICMR itself in these words:
“ICMR advocates that RT-PCR throat/nasal swab test is the best use for diagnosis of COVID 19. RT-PCR test detects the virus early and is the best strategy to identify and isolate the individual.
Several States have procured rapid antibody test kits and on their demand, ICMR has also provided these kits with clear instructions that they are to be used only for surveillance purposes,” the ICMR statement added.
It is true that there were complaints about quality of Chinese test kits from Europe, but note this: Reports claim that over 100 Chinese companies are selling coronavirus testing kits to Europe, but most are not licensed to sell in China, according to India Today.
Explaining why it was defending two private companies, the Chinese government said many countries had been buying medical equipment from the Chinese markets via commercial channels.
ICMR scientist Ganga khedkar’s earlier statement,as reported by Theprint, corroborated what China said above:
He further explained that the rapid antibody testing is conducted for “surveillance purposes” and to track the progression or regression of the disease in a particular geographical area.
“The antibody comes in the body after several days of pathogen invasion. It will be too late for diagnosis, if we relied on this testing,” the ICMR scientist said.
A rapid antibody test shows if a person once infected by the coronavirus has developed the immunity to it.
The kits were delayed due to reports of inaccuracies in their results. However, ICMR said “the kits will be used for mapping a trend, and not diagnosis. Even if the kit has an error rate, which is uniform across all results, we will be able to map the trend and use it for surveillance”.
“This is not a confirmatory test. The serological test is for the purpose of surveillance to generate data and understand whether people got exposed to the virus,” said Randeep Guleria, chairman of the high-level expert committee formed to review the testing strategy for Covid-19 by the Indian Council of Medical Research (ICMR). That was as early as March 27.
After being caught by the Court, ICMR in a hurry canceled the order for lakhs of kits, being supplied at a far lower landed cost of Rs 250 per kit. And ordered that kits already imported would be returned to the suppliers.
It is well known in pharma industry that when complaints arise, lakhs of doses of medicines are withdrawn by suppliers, as per agreed terms. The medical kits also are covered by similar rules.
We do not know what are the terms of this agreement; what if the loss would be, and who would bear the costs. The media is not investigating that. It is happy with fed news.
And reports of a brave face are there action would be taken for faulty kits, but no action against unscrupulous traders aided by ICMR, are contemplated.
And more than all, the surveillance and epidemiological purpose of the RAT Kits would be impaired, which is crucial for India at this stage.
India tested around 783 tests per million population as on May 3. The figure for Germany is 30400, Turkey 13177, Italy 34879, USA 20940, Sapin 32699. India is among the lowest in 20 countries worst affected, Hindustan Times reported May 3.
India badly needs to test on a large scale with RAT kits. There are no suppliers who can supply in a large scale and at a low price as China does.
India is projected by PM Modi and his troupe as a great power of 21st century, with hitech, satellites and nuclear weapons too. However, when it comes to smaller things of education and health, it is found wanting. Modi recently spoke of self-reliance, which was forgotten by Congress-led UPA as well BJP and NDA. It was he who subtly changed from Made in India to Make in India, which admittedly could be no more than assembling.
Why was hitech India forced to depend on foreign test kits?
New York Governor raised this question, saying it is a shame for USA to be found wanting in equipments and medicines. But no one raised this important question in India.
India tested around 783 tests per million population as on May 3. The figure for Germany is 30400, Turkey 13177, Italy 34879, USA 20940, Sapin 32699. India is among the lowest in 20 countries worst affected, Hindustan Times reported May 3. India badly needs to test with RAT kits.
India given its population needs huge capacity for testing. Covid-19 is new, but the world and India too are plagued for long by corona viruses, like Swine Flu, SARS. But it has few standard kits makers.
Haryana canceled import of China’s kits as they were too costly, said Health Minister Anil Vij, reported India Today April 22. It favored South Korea’s kit, of SD Biosensor , priced at Rs. 380, though they are being made (rather assembled) at Manesar plant near Delhi.
Three months after Covid-19 arrived in India, India is still lagging behind in its war-like efforts. livemint.com reported 01 May 2020 :
“In a setback, the first samples of state-owned HLL Lifecare Ltd’s indigenous rapid antibody test kits that was sent to Kerala has failed quality tests, three people in the know told Mint.
“Out of 100-odd samples, a first batch has failed the quality test. It had accuracy problems….Raw material for the kits were sourced from Canada and then assembled by HLL Lifecare in India. HLL Lifecare had won the tender by quoting the lowest bid at Rs. 336 per kit.(China kits’ landed cost Rs.245) …
The Indian firms — Gujarat-based Voxtur Bio, Delhi-based Vanguard Diagnostics and the government-owned HLL Lifecare — have been approved for supplying test kits. HLL plans to manufacture two million kits by 30 May, a report in Hindustan Times claims.
The other two firms depend on China for some components to manufacture these kits and an embargo on exports of these components has left their operations in limbo.
China’s statement needs to be taken seriously. They said:
Regarding the current issue occurred, we hope the Indian side could respect China’s goodwill and sincerity, strengthen communication timely with relevant Chinese companies based on facts, and resolve it reasonably and properly.
Annexure
China’s full statement of April 28 as given by republicworld.com :
Question: On April 27th, Indian Council of Medical Research (ICMR) announced that since some States have raised issues regarding the performance of rapid antibody test kits procured from Guangzhou Wondfo Biotech and Zhuhai Livzon Diagnostics, ICMR has evaluated the kits in field conditions, and the results have shown wide variation in their sensitivity despite early promise of good performance. In view of this, States are advised to stop using these kits procured from above mentioned companies and return them to be sent back to the suppliers. What’s your comment on this?
Answer: We are deeply concerned with the evaluation results and the decision made by Indian Council of Medical Research. China attaches great importance to the quality of exported medical products.
Recently, Chinese Embassy in India has maintained close contact with ICMR and the two Chinese companies to find out the real situation.
We have noticed that Guangzhou Wondfo Biotech and Zhuhai Livzon Diagnostics already issued statements on this issue. They both stressed that their COVID-19 antibody rapid test kits had obtained the certification from the National Medical Products Administration of China (NMPA), meet the quality standards of China and exported countries, and have also been validated and approved by ICMR through National Institute of Virology (NIV), Pune and considered as satisfactory products. The COVID-19 antibody rapid test kits produced by these two Chinese companies have been exported to and well recognized in many countries in Europe, Asia and Latin America.
We have also learned that there are strict requirements for the storage, transportation and use of COVID-19 antibody rapid test kits. Any operation which is not carried out by professionals in accordance with the product specifications will lead to the testing accuracy variations.
ICMR also made it clear that rapid antibody test kits should only be used for surveillance purposes instead of replacing RT-PCR test to diagnose and confirm the cases, and States are advised to strictly abide by the methods and purpose of the usage according to ICMR’s clear instructions.
Currently, many countries have been purchasing medical products through commercial channels from Chinese market. While satisfying domestic demands, we support export by companies with qualifications and credibility, and provide convenience in production, transport and customs clearance to facilitate foreign procurement and orderly exporting to countries including India.
Chinese relevant manufacturers have been working around the clock to try their best to provide medical supplies to help other countries fight the epidemic and protect people’s lives and health.
China not only sincerely supports India in its fight against Covid-19, but also takes concrete actions to help. The quality of medical products exported from China is prioritized. It is unfair and irresponsible for certain individuals to label Chinese products as “faulty” and look at issues with preemptive prejudice.
Regarding the current issue occurred, we hope the Indian side could respect China’s goodwill and sincerity, strengthen communication timely with relevant Chinese companies based on facts, and resolve it reasonably and properly.
Viruses are common enemy of mankind. Only by working together, can we win this battle against the epidemic. Since the outbreak of the epidemic, China and India have maintained close communication and cooperation on epidemic prevention and control.
Following India’s epidemic situation, China has been feeling the same, shared its experiences in epidemic prevention, control and treatment, and donated medical materials to India.
We will continue to support India’s efforts in fighting Covid-19, strengthen medical and health cooperation, and jointly work with India to overcome the difficulties at an early date, so as to safeguard the safety and health of our peoples as well as global and regional public health security
 Eurasia Press & News
Eurasia Press & News



